Protein Structure, Stability, and Function: From Bench to Model
We’ll explore how proteins are built, what keeps them in their functional shapes, and how we can study their stability both in the wet lab and using computational tools. Understanding these concepts is crucial for interpreting your experimental results and connecting them to the molecular level.
Learning Objectives:
- Identify and describe the four levels of protein structure.
- Recognize the major non-covalent and covalent forces that stabilize protein structures.
- Explain how environmental factors (heat, pH, salts) can affect protein stability and lead to denaturation.
- Understand the structural features contributing to the stability of model proteins like Lysozyme and Insulin.
- Relate common lab observations to underlying protein structural properties and how computational tools can provide further insights.
🧬 1. Protein Structure: Hierarchy and Forces
Proteins are the workhorses of the cell, performing a vast array of functions. Their function is intricately linked to their three-dimensional structure. This structure is organized in a hierarchical manner, with each level building upon the previous one. Various non-covalent and covalent interactions are the invisible architects that sculpt and stabilize these complex architectures.
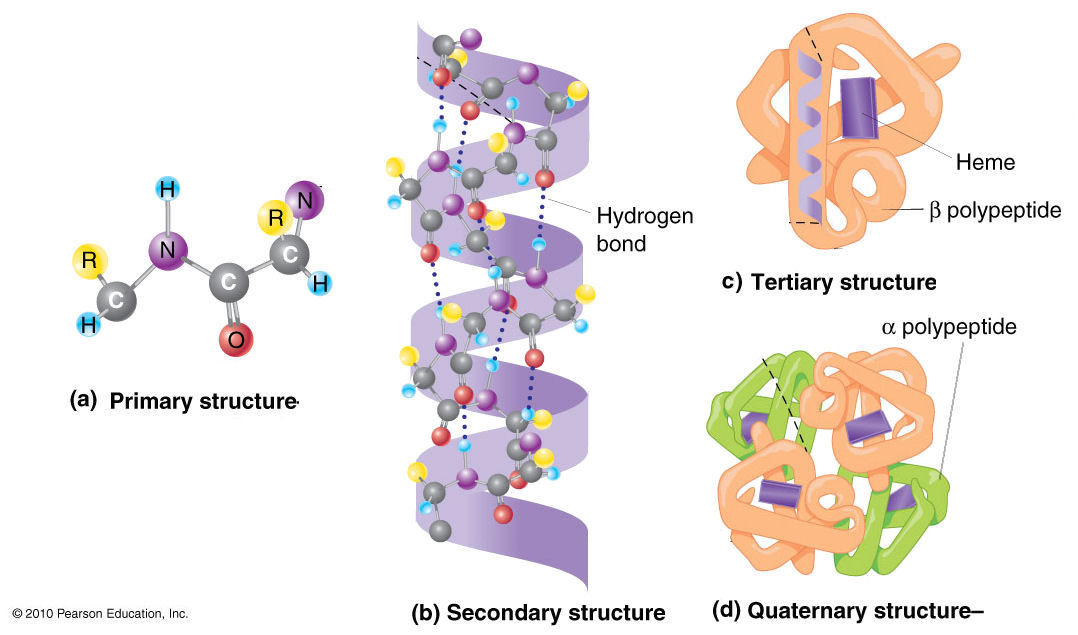
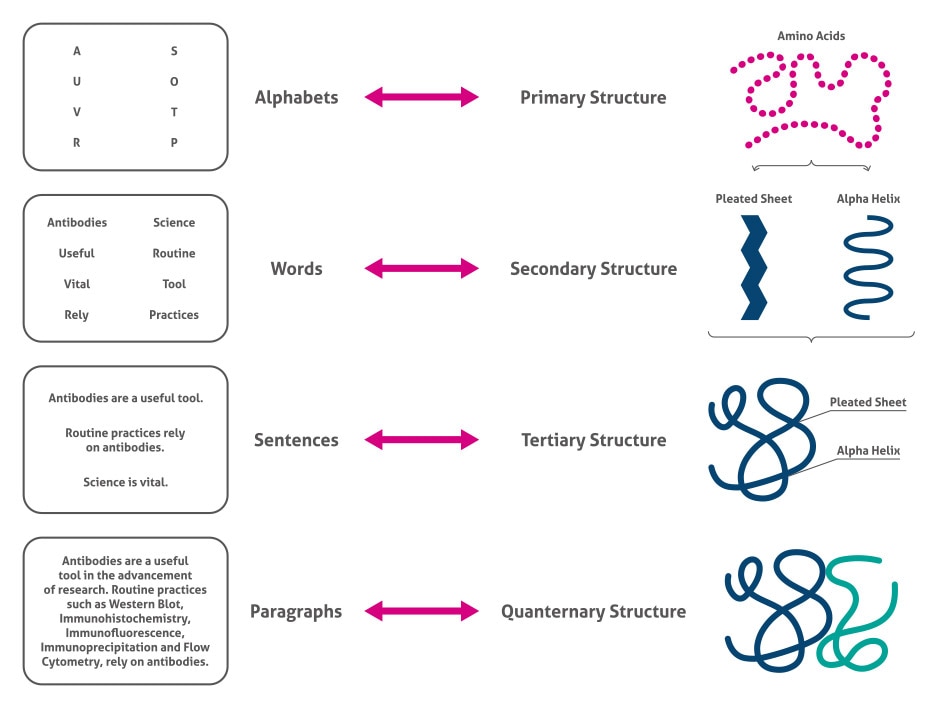
Let’s break down these levels:
| Level | Example | Description |
|---|---|---|
| Primary | GIVEQCCTSICSLYQLENYCN | The linear sequence of amino acids linked by peptide bonds. This sequence is determined by the gene encoding the protein. Think of it as the specific order of beads on a string. |
| Secondary | α-helix, β-sheet | Local, regular folding patterns stabilized by hydrogen bonds between the C=O and N-H groups of the peptide backbone. Common examples are the α-helix (a spiral) and β-sheet (a pleated structure). |
| Tertiary | Folded 3D domain | The overall three-dimensional shape of a single polypeptide chain. This intricate fold is driven by interactions between the amino acid side chains (R-groups), including hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds. This is when the “string of beads” folds into a specific, functional shape. |
| Quaternary | Hemoglobin (4 subunits) | The arrangement of multiple polypeptide chains (subunits) to form a functional protein complex. Not all proteins have quaternary structure. Hemoglobin, for instance, consists of four subunits working together. |
🛠 Tools for Exploring Structure:
- UniProt (Universal Protein Resource): A comprehensive database for protein sequence and functional information. You can find the primary structure (amino acid sequence) of virtually any known protein here.
- Try this: Go to UniProt and search for “human lysozyme” (P61626). Explore the “Sequence” section.
- AlphaFold DB (AlphaFold Protein Structure Database): Provides high-quality predictions of protein tertiary structures. Incredibly useful for proteins whose structures haven’t been experimentally determined.
- Try this: Search for “human lysozyme” on AlphaFold DB and see its predicted 3D fold.
- PDB (Protein Data Bank): A repository for experimentally determined 3D structures of proteins and nucleic acids. This is the primary source for high-resolution structural data.
- Try this: Search for PDB ID
1LYZ(hen egg-white lysozyme) on RCSB PDB.
- Try this: Search for PDB ID
- Mol* Viewer (pronounced MolStar): A powerful web-based tool for visualizing and analyzing molecular structures from PDB and AlphaFold DB. You can explore interactions, surfaces, and more.
- Try this: After finding
1LYZon the PDB, click the “3D View” or link to Mol* to interact with the structure. Try to identify α-helices and β-sheets.
- Try this: After finding
🔗 2. What Holds a Protein Together?
The intricate 3D structures of proteins are not just random tangles. They are precisely folded and maintained by a variety of chemical interactions. Understanding these forces is key to understanding protein stability and function.
Here’s a simplified interaction map showing key forces:
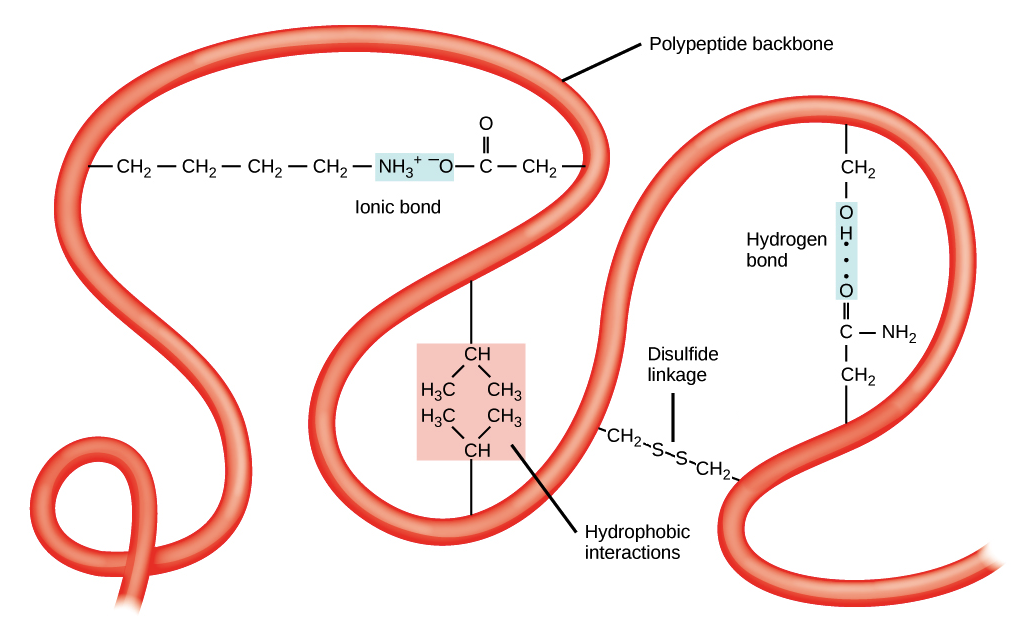
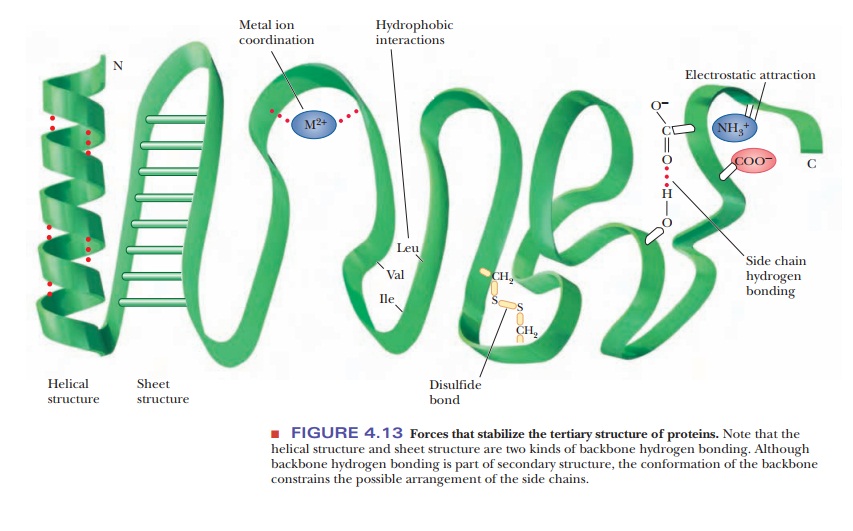
Let’s detail these interactions:
| Interaction Type | Description | Analogy | Affected By |
|---|---|---|---|
| Hydrogen Bonds | Weak electrostatic attractions between a hydrogen atom (bonded to N or O) and another electronegative atom (N or O). Think of them as tiny magnets. | Velcro strips – individually weak, but many together provide strong adhesion. | pH, temperature |
| Hydrophobic Effect | The tendency of nonpolar (water-fearing) side chains to associate with each other and minimize contact with water. This drives them to the protein’s core. | Oil droplets clumping together in water. | Detergents, heat, organic solvents |
| Ionic Interactions (Salt Bridges) | Attractions between oppositely charged groups (e.g., positively charged Lysine and negatively charged Aspartate). These are like stronger magnets than H-bonds. | Two magnets attracting each other. | pH, salt concentration |
| Disulfide Bonds | Covalent bonds formed between the sulfur atoms of two Cysteine residues (-S-S-). These are strong “staples” that lock parts of the protein together. | Permanent stitches or staples holding fabric pieces together. | Redox agents (reducing/oxidizing) |
| Van der Waals Forces | Weak, short-range attractive forces between all atoms when they are very close. Result from temporary fluctuations in electron distribution. Important for the tight packing of atoms in the protein core. | The subtle stickiness of cling film when pressed together. | Heat, conformational changes |
🧪 3. Denaturation & Stability: What the Lab Shows
A protein’s function depends on its specific 3D structure. When this structure is disrupted, the protein is said to be denatured, and it usually loses its activity. Various physical and chemical factors can cause denaturation by breaking the interactions we just discussed.
-
Thermal Denaturation (Heat):
- What happens? Increased kinetic energy (heat) disrupts weaker interactions like hydrogen bonds and Van der Waals forces. The hydrophobic core can be exposed, leading to aggregation and precipitation.
- Analogy: Shaking a delicate house of cards too vigorously will cause it to collapse.
- Lab observation: Protein solution becomes cloudy or forms a precipitate upon heating.
-
pH Changes:
- What happens? The pH of the solution affects the ionization state (charge) of acidic (e.g., Asp, Glu) and basic (e.g., Lys, Arg, His) amino acid side chains. Drastic pH changes can alter or break ionic interactions and hydrogen bonds that rely on specific charge states.
- Analogy: Changing the polarity of magnets can cause them to repel instead of attract.
- Lab observation: Protein solubility changes (may precipitate or dissolve) as pH is varied.
-
Salt Effects (Ionic Strength):
- What happens? Salts can have complex effects.
- At low concentrations, salts can sometimes stabilize proteins by shielding unfavorable charge-charge repulsions (salting-in).
- At high concentrations, salts can compete with protein groups for water molecules, disrupting the hydration shell and hydrophobic interactions, leading to aggregation and precipitation (salting-out). This is often used for protein purification.
- Lab observation: Adding salt might initially increase solubility, but very high salt concentrations often cause precipitation.
- What happens? Salts can have complex effects.
-
Detergents:
- What happens? Detergents are amphipathic molecules (having both polar and nonpolar parts). They can disrupt hydrophobic interactions by solvating nonpolar regions, effectively “turning the protein inside out.”
- Lab observation: Often used to solubilize membrane proteins or denature proteins for techniques like SDS-PAGE.
-
Reducing Agents (e.g., β-mercaptoethanol, DTT):
- What happens? These chemicals specifically break disulfide bonds.
- Lab observation: Loss of structural integrity in proteins heavily reliant on disulfide bonds for stability.
-
Metal Binding:
- What happens? Some proteins require metal ions (e.g., Zn²⁺, Mg²⁺, Fe²⁺/³⁺) for their structure or function. These metals often bind to specific amino acid side chains (like Histidine, Cysteine) in defined pockets.
- Lab observation: Adding a specific metal ion might be necessary for activity, or its removal (using chelators like EDTA) might lead to loss of function or denaturation. Sometimes, metal binding causes a color change.
Wet lab experiments, like the ones you might perform, allow you to probe these properties. By observing changes in solubility, color, or using spectrophotometry to monitor unfolding, you can link a protein’s behavior to its underlying structural characteristics.
🧠 4. Case Study: Lysozyme – A Model of Stability
Lysozyme is a classic example used in protein biochemistry studies, often found in egg white, tears, and saliva. It’s a relatively small, robust enzyme.
- Function: It acts as an antibacterial agent by breaking down peptidoglycans, which are major components of bacterial cell walls.
- Structure: Lysozyme has a compact, globular structure rich in α-helices and some β-sheet regions. A key feature contributing to its stability is the presence of four intramolecular disulfide bonds.
- 🔗 PDB ID:
1LYZ(Hen Egg White Lysozyme) - Explore it on RCSB PDB - 🔗 AlphaFold Model: AF-P00698-F1 (Human Lysozyme, for comparison)
- 🔗 PDB ID:
- Why is it so stable?
- Disulfide Bridges: These covalent cross-links act like “safety pins,” holding different parts of the polypeptide chain together, making it much harder to unfold.
- Tight Packing: Its compact structure means a well-organized hydrophobic core and numerous Van der Waals interactions, contributing to overall stability.
🧪 Lab Connection: In experiments involving heat fractionation (separating proteins based on their heat stability), lysozyme often remains soluble and active even at elevated temperatures where many other proteins would denature and precipitate. Your lab experiment might demonstrate this remarkable heat resistance!
💉 5. Case Study: Insulin – Small Protein, Big Implications
Insulin is a crucial peptide hormone that regulates glucose metabolism in the body. Despite its small size, its structure is precise and essential for its potent biological effect.
- Structure: Mature human insulin consists of two polypeptide chains: the A chain (21 amino acids) and the B chain (30 amino acids). These chains are linked together by two inter-chain disulfide bonds. Additionally, the A chain contains one intra-chain disulfide bond.
- Small but Precise: Even minor alterations or misfolding can lead to a loss of activity, highlighting the critical nature of its specific 3D conformation for binding to its receptor.
- Sensitivity:
- Heat: Insulin is relatively heat-sensitive and can denature easily if not stored properly. Unlike some larger, more robust proteins, it doesn’t have extensive hydrophobic cores or the same degree of structural reinforcement found in proteins like lysozyme.
- Glycosylation: Insulin is not glycosylated (doesn’t have sugar chains attached). Glycosylation can sometimes increase the stability and solubility of proteins, but insulin relies solely on its amino acid sequence and disulfide bonds for its structure.
💭 Modeling Note for Insulin: When you explore insulin’s structure using Mol* or other viewers, pay close attention to:
- The inter-chain disulfide bonds: How do they hold the A and B chains together?
- The intra-chain disulfide bond within the A chain: How does it contribute to the local fold of the A chain? Insulin is an excellent example of how structure-function relationships are tightly regulated, even in small peptides, and how disulfide bonds can be critical for maintaining the integrity of multi-chain proteins.
🔄 6. Bridging Lab to Modeling: What to Observe & Why
The beauty of biochemistry lies in connecting what you observe in a test tube (your lab experiment) with what’s happening at the molecular level (protein structure and interactions). Computational modeling tools can help bridge this gap.
Here’s how some common lab observations might relate to a protein’s structural features, and what you could investigate using modeling:
| Lab Observation | What It Suggests About the Protein | Modeling Connection / What to Look For |
|---|---|---|
| Precipitation after gentle heating (e.g., 50-60°C) | Protein is relatively heat-labile (sensitive to heat). | Likely has a less compact fold, fewer stabilizing disulfide bonds, or a more exposed hydrophobic core. Check its AlphaFold predicted pLDDT score (confidence); lower scores in regions might indicate flexibility. |
| Remains soluble and active at 80°C | Protein is heat-stable and likely compact. | Look for features like disulfide bonds, extensive hydrogen bond networks, a well-packed hydrophobic core, or a high proportion of rigid secondary structures (e.g., β-sheets). |
| Binds a specific metal ion (e.g., iron) and shows a color shift | Possesses a specific binding pocket for that metal. | Use Mol* to examine the PDB/AlphaFold structure. Look for clusters of potential metal-coordinating residues (His, Cys, Asp, Glu, main chain carbonyls). Tools like CASTp can predict pockets. |
| Solubility drastically changes with pH shifts around neutral pH | Surface charges are critical for its solubility; likely has many ionizable residues on its surface. | In Mol*, display the electrostatic surface potential. Observe the distribution of positive and negative charges. How does this change if you consider different protonation states (though this is advanced)? |
| Precipitates at high salt concentrations | Undergoes “salting-out”; its hydration shell is disrupted. | Examine surface hydrophobicity. Proteins with large hydrophobic patches might be more prone to salting out. (This is more about general properties than a specific structural motif). |
| Loses activity when a reducing agent is added | Disulfide bonds are essential for its active conformation. | Identify Cysteine residues in Mol*. Are they close enough to form disulfide bonds? (PDB files often explicitly state disulfide bonds). How would breaking them impact the overall fold? |
🧰 7. Tools to Use During/After the Lab
Here’s a quick reference for some bioinformatics tools that will be invaluable as you analyze your proteins and lab results:
| Tool Name | Use Case | Link | Pro Tip / Example Task |
|---|---|---|---|
| UniProt | Get canonical sequences, functional annotations, variant info, PTMs. | uniprot.org | Search for your protein. Check the “Function” and “Subcellular location” sections. Are there any disease associations listed under “Pathology & Biotech”? |
| AlphaFold DB | Obtain high-quality predicted 3D structures. | alphafold.ebi.ac.uk | Check the pLDDT score (per-residue confidence). Regions with low pLDDT (< 70) might be intrinsically disordered or flexible. |
| RCSB PDB | Access experimentally determined 3D structures. | rcsb.org | Look for bound ligands, metal ions, or other molecules in the experimental structure. Check the “Experimental Details” section to understand how the structure was solved. |
| Mol* Viewer | Visualize and analyze 3D structures (from PDB or AlphaFold). Explore interactions, surfaces, sequences. | molstar.org/viewer | Load a structure (e.g., 1LYZ). Try coloring by secondary structure. Use the “measurements” tool to check distances between atoms potentially involved in an interaction. |
| CASTp (Computed Atlas of Surface Topography of proteins) | Identify and measure pockets and cavities on protein surfaces. Useful for predicting ligand binding sites. | sts.bioe.uic.edu/castp | Upload a PDB file (or use a PDB ID) to find potential active sites or binding pockets. Note the area and volume of the largest pockets. |
| PROSITE | Scan protein sequences for known functional motifs, patterns, and profiles. | prosite.expasy.org | Input your protein sequence (from UniProt) to see if it contains any known functional sites (e.g., enzyme active site patterns, binding motifs). |
🌟 Conclusion
Understanding protein structure, the forces that govern stability, and how experimental conditions affect them is fundamental to biochemistry. By combining wet lab observations with insights from computational tools, you can gain a much deeper appreciation for how these remarkable molecules function.
As you proceed with your lab work, keep these concepts in mind. Think about:
- What does this observation (e.g., precipitation, color change, loss of activity) tell me about my protein’s structure and stability?
- How do the specific amino acids in its sequence contribute to these properties?
- How can I use tools like PDB, AlphaFold, and Mol* to visualize and understand these properties at a molecular level?
Good luck with your experiments!
- Resources
- API
- Sponsorships
- Open Source
- Company
- xOperon.com
- Our team
- Careers
- 2025 xOperon.com
- Privacy Policy
- Terms of Use
- Report Issues