Protein-Protein Interactions: The Social Network of the Cell
Introduction: The Secret Life of Proteins
In the bustling city of the cell, proteins are the primary workers. They are enzymes, structural supports, messengers, and motors. But here’s a crucial secret: proteins rarely act alone. They form a vast and dynamic social network, constantly interacting with each other to carry out their functions. This network of Protein-Protein Interactions (PPIs) is the backbone of almost every cellular process, from DNA replication to signal transduction and metabolic pathways.
Understanding these interactions is fundamental to systems biology, disease research, and drug discovery. This lesson will guide you through the types of PPIs, the experimental and computational methods used to discover them, and the databases where this information is stored.
1. Types of Protein-Protein Interactions
Not all interactions are created equal. We can broadly classify them into two main categories: functional and physical.
1.1 Functional Interactions
A functional interaction means that two or more proteins work together to accomplish a task, but they don’t necessarily have to be in direct physical contact at the same time.
Analogy: Think of a relay race team. Each runner is part of the same functional unit (the team) and contributes to the overall goal (finishing the race), but only two runners interact at any given moment during the baton handoff.
A classic example is a metabolic pathway, like the TCA (Krebs) Cycle. The enzymes in this cycle are functionally linked because the product of one enzyme becomes the substrate for the next. While they may not all be bound together, their activities are sequentially coordinated.
1.2 Physical Interactions
This is what we typically think of when we say “PPI”: a direct, physical binding between two or more proteins. These can be further divided based on how long they last.
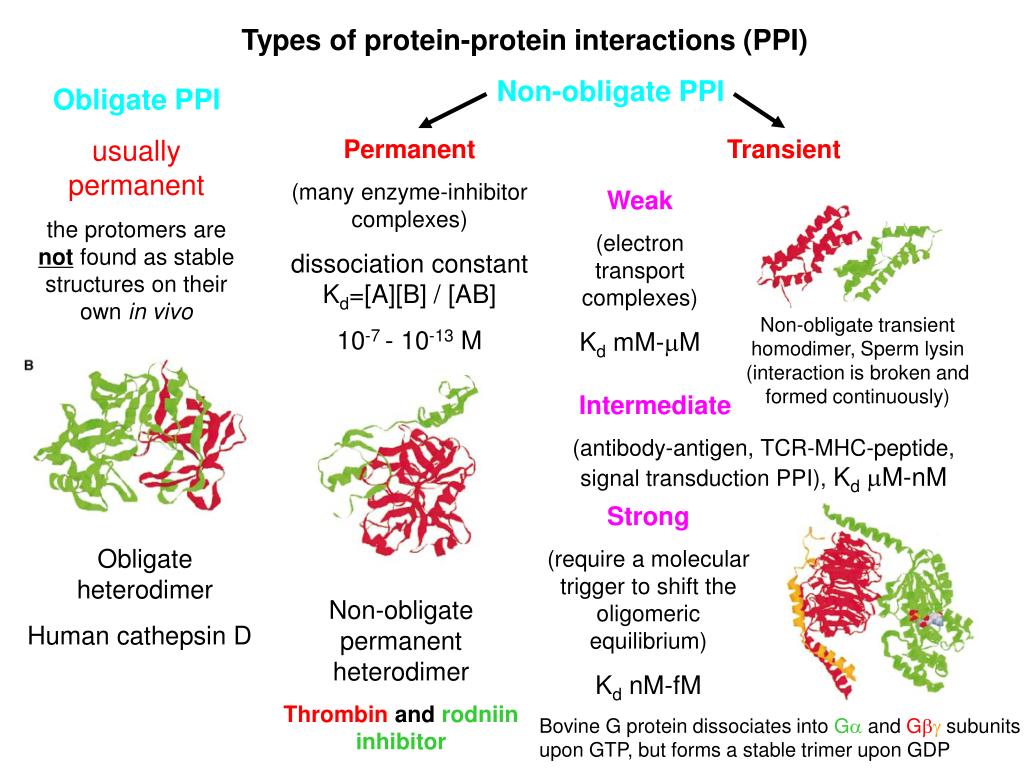
a. Stable (Permanent) Interactions
These are long-lasting interactions where proteins come together to form a multi-subunit complex that functions as a single machine. The individual proteins (subunits) may even be unstable on their own.
- Example: Hemoglobin. This protein, which carries oxygen in your blood, is a stable complex of four subunits (two alpha and two beta chains). These four proteins are almost always found together.
- Analogy: A band that always writes, rehearses, and performs together as a single unit.
b. Transient Interactions
These are temporary, “touch-and-go” interactions that form and break apart depending on specific signals or cellular conditions. They are often key to signaling pathways and regulation.
- Example: Cell Survival Signaling. The protein Akt1 phosphorylates the protein BAD. This phosphorylation causes BAD to bind to another protein, 14-3-3. This transient interaction prevents BAD from causing cell death, thus promoting cell survival. When the signal is gone, they dissociate.
- Analogy: A freelance consultant hired for a specific, short-term project. They work closely with the team, then leave once the job is done.

2. The Diversity of Physical Interactions
To add more detail, we can classify physical interactions using two axes: structural similarity (are the proteins identical?) and structural stability (can the proteins exist alone?).
-
Structural Similarity
- Homo-oligomer: A complex made of identical protein subunits (e.g., two copies of protein A form a homodimer).
- Hetero-oligomer: A complex made of different protein subunits (e.g., protein A binds to protein B to form a heterodimer).
-
Structural Stability
- Obligate Interaction: The subunits are unstable on their own and must form a complex to fold correctly and be stable. The complex is the only functional form found in the cell.
- Non-obligate Interaction: The subunits are stable on their own and can exist and function independently, but they can also come together to form a complex. Most transient interactions are non-obligate.
These classifications can be combined. For example, hemoglobin is an obligate hetero-tetramer.
3. How Do We Find PPIs? Experimental Methods
Detecting the invisible dance of proteins requires a sophisticated toolkit. Scientists have developed a wide array of methods, each with its own strengths and weaknesses. Before we explore the specific techniques, we need to understand some essential terminology and a core principle that underpins many of these experiments.
The Bait and Prey Principle: Fishing for Protein Partners
Many of the most powerful PPI detection methods are based on a simple but effective “fishing” analogy known as the Bait and Prey Principle.
Imagine you want to know what kind of fish a specific piranha eats. The piranha is your known entity. The insects, smaller fish, and other creatures it might eat are the unknowns. In this scenario:
- The Bait (B): Your protein of interest (the piranha). You know its identity and will use it to “fish.” In experiments, the bait is often tagged or modified so you can easily track it and pull it out of a mixture.
- The Prey (P): The potential, unknown binding partners (the insects). They exist in a complex environment, like a “lake” full of thousands of other proteins (a cell lysate).
- The Experiment: The process of allowing the bait to interact with the pool of potential prey and then selectively isolating the bait. If a prey protein was bound to the bait, it gets isolated too.
- The Discovery: By analyzing what you caught along with your bait, you identify the prey.

This principle turns a needle-in-a-haystack problem into a targeted search. You aren’t just randomly looking for any two interacting proteins; you are specifically asking, “What does my protein interact with?”
Now, let’s explore how this principle, along with other physical measurements, is applied in various experimental methods. These are broadly categorized into Biophysical, Biochemical, and Genetic approaches.

3.1 Biophysical Methods (Optional)
Biophysical methods measure changes in the physical properties of molecules upon binding. They are performed in vitro with purified proteins and are the gold standard for getting quantitative data like binding strength (affinity) and kinetics (on/off rates). Their primary focus is on measuring the physical change itself.
A) Fluorescence Polarization (FP)
- Principle: Measures the speed of molecular rotation. A small, fluorescently-labeled molecule (the “prey”) tumbles rapidly, depolarizing light. When it binds to a large protein (the “bait”), the complex tumbles much more slowly, and the emitted light stays polarized. This change indicates binding.
B) Surface Plasmon Resonance (SPR)
- Principle: Detects changes in mass on a sensor surface. The “bait” protein is immobilized on a gold chip. As a solution containing the “prey” protein flows over, any binding increases the mass on the surface, which is detected in real-time as a change in the refractive index.
C) Isothermal Titration Calorimetry (ITC)
- Principle: Directly measures the tiny amount of heat released or absorbed when two molecules bind. A solution of “prey” protein is slowly injected into a chamber containing the “bait” protein, and the resulting heat change is precisely measured to determine the thermodynamics of the interaction.
D) Microscale Thermophoresis (MST)
- Principle: Measures the change in a molecule’s movement along a microscopic temperature gradient. The movement of a fluorescently-labeled molecule (“prey”) changes when it binds to a partner (“bait”) due to differences in size, charge, and hydration shell.
3.2 Biochemical Methods (Optional)
These methods are classic applications of the Bait and Prey principle, using molecular tags, antibodies, or engineered reporters to detect interactions.
Proximity-Based Assays: Is My Bait Near My Prey?
These techniques generate a signal only when the bait and prey proteins are brought very close to each other by a binding event.
-
A) FRET & B) BRET: The bait is fused to a donor molecule (CFP or Luciferase) and the prey is fused to an acceptor molecule (YFP). Energy transfer (and the resulting acceptor signal) occurs only when the bait and prey bind, bringing the donor and acceptor into close proximity.
-
C) AlphaScreen: The bait is attached to a Donor bead and the prey to an Acceptor bead. A signal is generated only when the bait-prey interaction brings the beads close enough for a singlet oxygen molecule to travel from one to the other.
-
D) Protein-fragment Complementation Assay (PCA): A reporter protein (e.g., GFP) is split into two halves. The bait is fused to one half, and the prey to the other. A signal is produced only when the bait and prey interact, reassembling the two halves into a functional reporter protein.
Affinity Purification Assays: The “Fishing” Expeditions
These methods physically isolate the “bait” protein and its bound “prey” from a complex mixture.
-
E) Pull-down Assay & F) Tandem Affinity Purification (TAP): A tag (like GST or a TAP-tag) is attached to the bait protein. This tagged bait is used to “pull down” its binding partners (prey) from a cell lysate using beads that recognize the tag. TAP uses two successive purification steps for higher specificity.
-
G) Co-Immunoprecipitation (Co-IP): This is the classic in vivo bait-and-prey method. An antibody specific to the native bait protein is used to pull it out of the cell lysate. Any prey proteins that are naturally bound to it “co-precipitate” and are identified.
-
H) ELISA: The bait protein is stuck to the bottom of a plate well. A lysate containing the potential prey is added. If the prey binds, it gets captured in the well and is then detected using a specific antibody.
Proximity Ligation Assay (PLA)
Antibodies for the bait and prey are used. If these proteins are close, attached DNA strands can be ligated and amplified, creating a fluorescent spot that signals an interaction.
3.3 Genetic Methods
These methods manipulate cellular systems, fully embracing the bait-and-prey model to produce a clear, readable output.
-
A) Phage Display: The bait protein is immobilized. A huge library of phages, each displaying a different potential prey protein, is washed over it. Only the phages displaying a prey that binds to the bait will stick.
-
B) The Yeast Two-Hybrid (Y2H) System: The bait is fused to a DNA-Binding Domain (BD). A library of prey proteins is fused to an Activation Domain (AD). An interaction between bait and prey brings the BD and AD together, activating a reporter gene.
-
C) Protein Microarray: Here, the roles are reversed but the principle is the same. Thousands of different prey proteins are spotted and immobilized on a slide. A fluorescently labeled bait protein is then washed over the slide to see which spots it binds to.
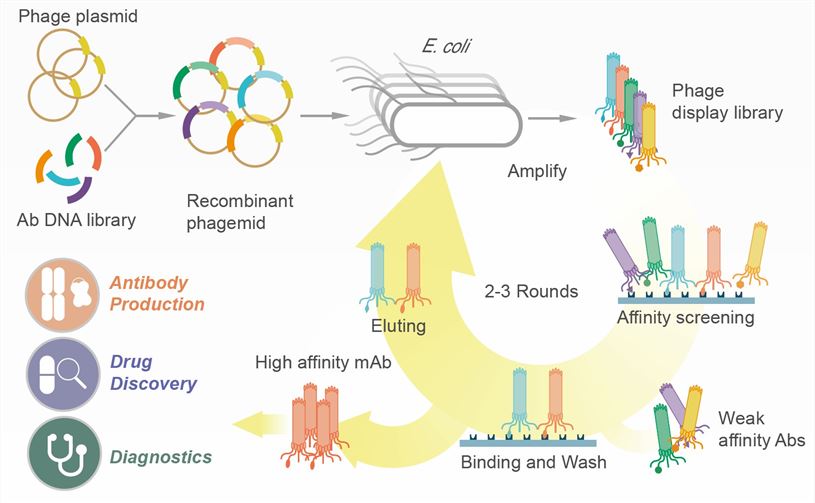 Figure. Phage Display
Figure. Phage Display
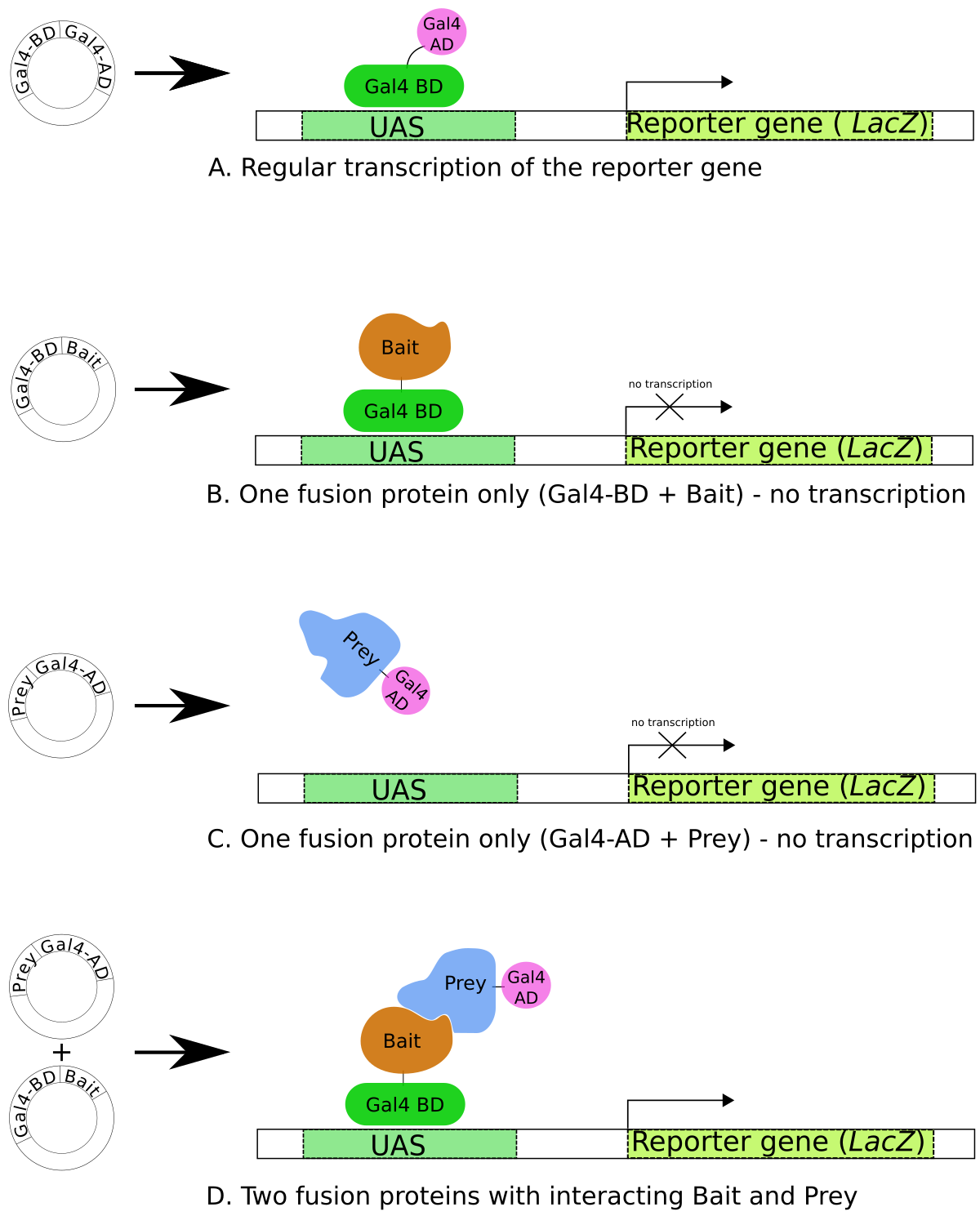 Figure. The Yeast Two-Hybrid (Y2H) System
Figure. The Yeast Two-Hybrid (Y2H) System
4. Computational Methods for Predicting PPIs
Running experiments can be slow and expensive. Computational methods allow us to predict potential PPIs from genomic and other data, helping to prioritize targets for experimental validation. These methods often predict functional associations, which can then be investigated for physical interaction.
Key computational approaches include:
- Gene Co-expression: If two genes are consistently turned on and off at the same times across different conditions, their protein products might be functionally related.
- Genomic Context (Gene Neighborhood): In bacteria, genes whose products are part of the same complex or pathway are often located next to each other in the genome (operons).
- Phylogenetic Profiling: If two proteins are consistently both present or both absent across a wide range of species, they likely have a functional link. A protein cannot function without its essential partner.
- Sequence Co-evolution: If a mutation in one protein is compensated by a mutation in another protein over evolutionary time, it suggests they are in physical contact. The mutations preserve the fit between the two proteins.
- Structure-based Docking: If the 3D structures of two proteins are known, we can use computer algorithms to predict if and how they might fit together, like a 3D puzzle.

5. PPI Databases: The Global Interactome Map
Over decades, researchers have generated vast amounts of PPI data. This information is collected and curated in public databases. These are essential resources for any biologist. They allow you to look up a protein of interest and see its known interaction partners.
Analogy: If proteins have a social network, these databases are their LinkedIn or Facebook, showing all their documented connections.
Some of the most prominent PPI databases include:
- BioGRID: (Biological General Repository for Interaction Datasets) - A comprehensive database of protein and genetic interactions.
- IntAct: A major, open-source database curated at the European Bioinformatics Institute (EBI).
- STRING: Focuses not just on physical interactions but on a broad network of functional associations, scoring them based on different types of evidence (experimental, co-expression, text mining, etc.).
- MINT: (Molecular INTeraction database)
- DIP: (Database of Interacting Proteins)

Conclusion
Protein-protein interactions are the threads that weave together the complex tapestry of life. By understanding the different types of interactions and the array of experimental and computational tools at our disposal, we can begin to map out the intricate cellular machinery. No single method is perfect; a combination of approaches, from high-throughput screens to quantitative biophysics and computational predictions, is necessary to build a confident and detailed picture of the cellular interactome. As a bioinformatician, your ability to navigate, interpret, and integrate this data will be a critical skill.
- Resources
- API
- Sponsorships
- Open Source
- Company
- xOperon.com
- Our team
- Careers
- 2025 xOperon.com
- Privacy Policy
- Terms of Use
- Report Issues