C6: Understanding Enzymes - Nature's Catalysts
Welcome to your pre-lab reading for C6: Enzymes! Enzymes are essential biological molecules that play a critical role in virtually all processes within living organisms. This guide will introduce you to what enzymes are, how they work, and the factors that can influence their activity. Understanding these concepts will be crucial for your upcoming lab session.
1. What are Enzymes?
Enzymes are biological catalysts. This means they speed up biochemical reactions without being consumed or changed in the process. Most enzymes are proteins, and their specific three-dimensional structure is vital for their function.
Think of them as tiny, highly efficient helpers in your cells, facilitating reactions that would otherwise happen too slowly to sustain life. From digesting your food to synthesizing DNA, enzymes are involved every step of the way.
1.1. The Active Site and Substrates
Each enzyme has a unique region called the active site. This is a specific pocket or groove on the enzyme’s surface where the substrate – the molecule(s) the enzyme acts upon – binds.
The shape and chemical properties of the active site are complementary to the substrate, much like a lock and key. However, a more accurate model is the induced-fit model, where the active site can undergo slight conformational changes upon binding to the substrate to achieve a tighter fit.
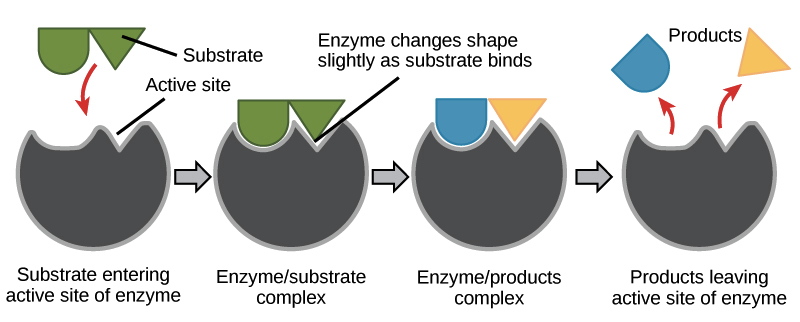 Figure: Enzyme-Substrate Interaction.
Figure: Enzyme-Substrate Interaction.
Once the substrate binds to the active site, it forms an enzyme-substrate complex (ES complex). Within this complex, the enzyme facilitates the conversion of the substrate(s) into product(s). After the reaction, the products are released, and the enzyme is free to bind to another substrate molecule.
The general sequence is: Enzyme (E) + Substrate (S) ⇌ Enzyme-Substrate Complex (ES) → Enzyme (E) + Product (P)
2. How Do Enzymes Speed Up Reactions? Lowering Activation Energy
Chemical reactions require a certain amount of energy to get started. This initial energy investment is called the activation energy (Ea). Enzymes work by lowering the activation energy needed for a reaction to occur.
Imagine trying to push a boulder over a hill. The hill represents the activation energy. An enzyme acts like a tunnel through the hill, providing an easier pathway that requires less energy to get the boulder (substrate) to the other side (product).
 Figure: Effect of an enzyme on activation energy. The enzyme provides an alternative reaction pathway with a lower activation energy (green curve) compared to the uncatalyzed reaction (red curve).
Figure: Effect of an enzyme on activation energy. The enzyme provides an alternative reaction pathway with a lower activation energy (green curve) compared to the uncatalyzed reaction (red curve).
By lowering the activation energy, enzymes significantly increase the rate at which reactions proceed.
3. Factors Affecting Enzyme Activity
The rate of an enzyme-catalyzed reaction can be influenced by several factors. In your lab, you will likely investigate some of these.
3.1. Temperature
- Effect: Generally, increasing the temperature increases the rate of an enzyme-catalyzed reaction, up to a certain point. This is because higher temperatures lead to more kinetic energy, resulting in more frequent collisions between enzyme and substrate molecules.
- Optimum Temperature: Each enzyme has an optimum temperature at which it functions most effectively.
- Denaturation: Beyond the optimum temperature, the enzyme’s structure begins to break down or denature. The weak bonds maintaining the enzyme’s 3D shape (especially the active site) are disrupted, leading to a loss of function. This is usually irreversible.
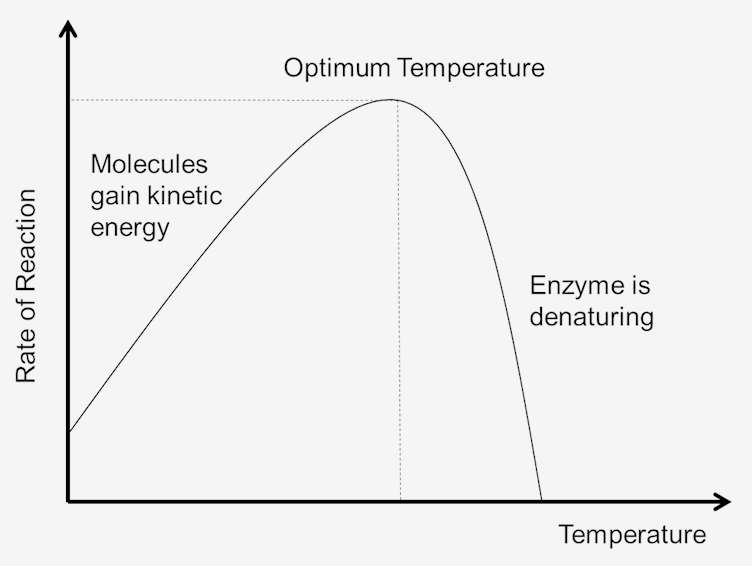 Figure: Typical effect of temperature on enzyme activity.
Figure: Typical effect of temperature on enzyme activity.
3.2. pH
- Effect: pH measures the acidity or alkalinity of a solution. Changes in pH can affect the ionization of amino acid R-groups in the enzyme, particularly those in the active site. This can alter the enzyme’s shape and its ability to bind the substrate.
- Optimum pH: Each enzyme has an optimum pH range where its activity is maximal.
- Denaturation: Extreme pH values (too acidic or too alkaline) can cause denaturation and irreversible loss of enzyme activity.
 Figure: Typical effect of pH on enzyme activity.
Figure: Typical effect of pH on enzyme activity.
3.3. Substrate Concentration
- Effect: If the enzyme concentration is kept constant, increasing the substrate concentration will increase the reaction rate. This is because there are more substrate molecules available to collide with enzyme active sites.
- Saturation Point: However, the rate will not increase indefinitely. Eventually, a point is reached where all enzyme active sites are occupied (saturated) with substrate. At this saturation point, the reaction rate plateaus, and adding more substrate will not significantly increase the rate.

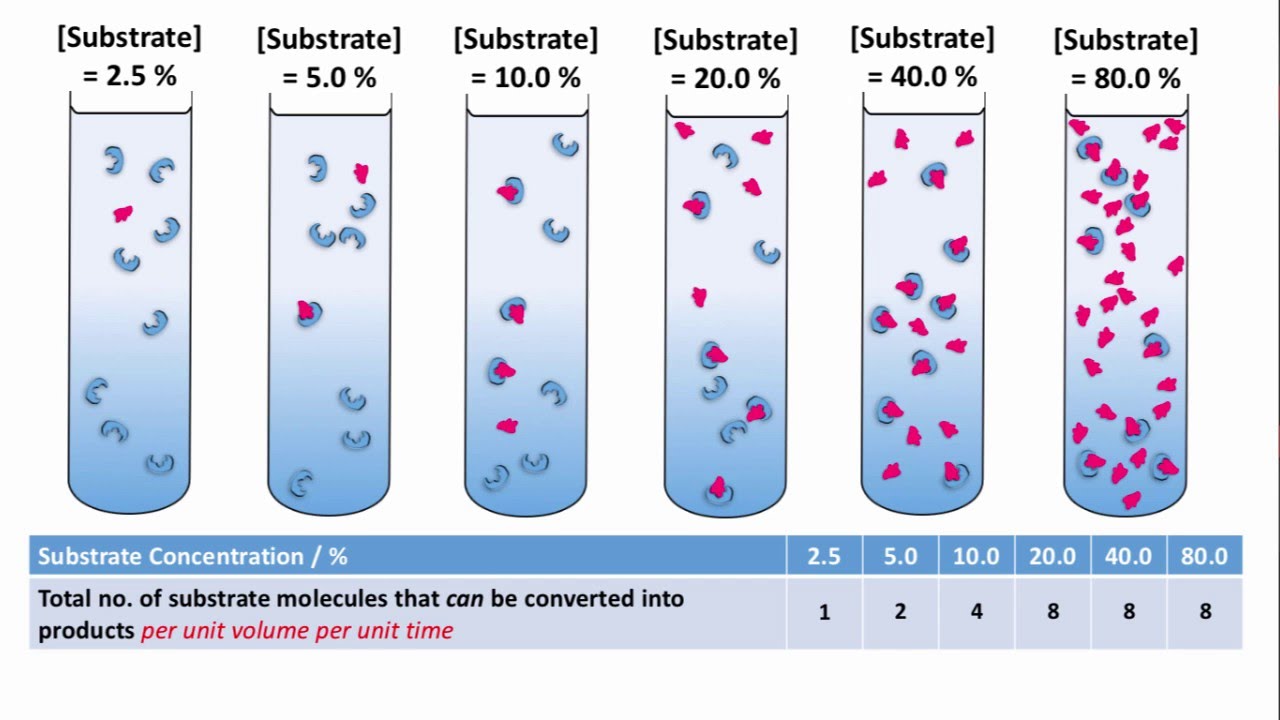 Figure: Typical effect of substrate concentration on enzyme reaction rate (assuming constant enzyme concentration).
Figure: Typical effect of substrate concentration on enzyme reaction rate (assuming constant enzyme concentration).
3.4. Enzyme Concentration
- Effect: If there is an excess of substrate, increasing the enzyme concentration will increase the reaction rate proportionally. More enzyme molecules mean more active sites available to process the substrate.
Watch this Video for a Recap:
This video provides a good overview of enzymes and how they work:
4. Enzyme Nomenclature
Enzymes are often named by adding the suffix “-ase” to the name of their substrate or the type of reaction they catalyze. For example:
- Lactase breaks down lactose.
- Lipase breaks down lipids (fats).
- DNA Polymerase synthesizes DNA polymers.
- Catalase catalyzes the decomposition of hydrogen peroxide.
6. Test Your Understanding
Let’s review some key concepts.
7. Conclusion
Enzymes are remarkable biological machines crucial for life. Understanding their structure, how they lower activation energy, and the factors that influence their activity will provide a solid foundation for your experiments in the lab. Pay close attention to experimental design and precise measurements to observe these principles in action!
Good luck with your lab!
- Resources
- API
- Sponsorships
- Open Source
- Company
- xOperon.com
- Our team
- Careers
- 2025 xOperon.com
- Privacy Policy
- Terms of Use
- Report Issues