C3: Introduction to Microscopy
Welcome to the microscopy lab! The microscope is one of the most important and frequently used tools in biology. It allows us to enter a world otherwise hidden from our naked eyes, revealing the intricate details of cells, tissues, and microorganisms. This pre-lab reading will guide you through the basics of microscopy to prepare you for your hands-on experience.
1. What is Microscopy?
Microscopy is the science of investigating small objects and structures using a microscope. An instrument that magnifies objects too small to be seen with the naked eye is called a microscope. In biology, microscopy is essential for:
- Observing cell structure (cytology).
- Examining tissues (histology).
- Identifying microorganisms (microbiology).
- Understanding fundamental biological processes.
A Brief Look Back (Optional)
The invention of the microscope revolutionized biology. While you don’t need to memorize dates, understanding its development highlights its impact.
- Late 1500s: Zacharias Janssen and his father Hans are credited with making the first compound microscope.
- Mid-1600s: Antonie van Leeuwenhoek, a Dutch linen merchant, crafted simple microscopes with powerful single lenses and was the first to observe and describe bacteria, yeast, and the circulation of blood corpuscles in capillaries. He is often called the “Father of Microbiology.”
- Late 1600s: Robert Hooke, an English scientist, observed thinly sliced cork using a compound microscope and coined the term “cell” after the small rooms monks lived in.
2. The Compound Light Microscope
In this lab, you will be using a compound light microscope. It’s called “compound” because it uses two sets of lenses (ocular and objective lenses) to magnify an object. It’s called a “light” microscope because it uses visible light to illuminate the specimen.
Parts of the Compound Light Microscope
Familiarize yourself with the parts of the microscope. Knowing their names and functions is crucial for proper use.
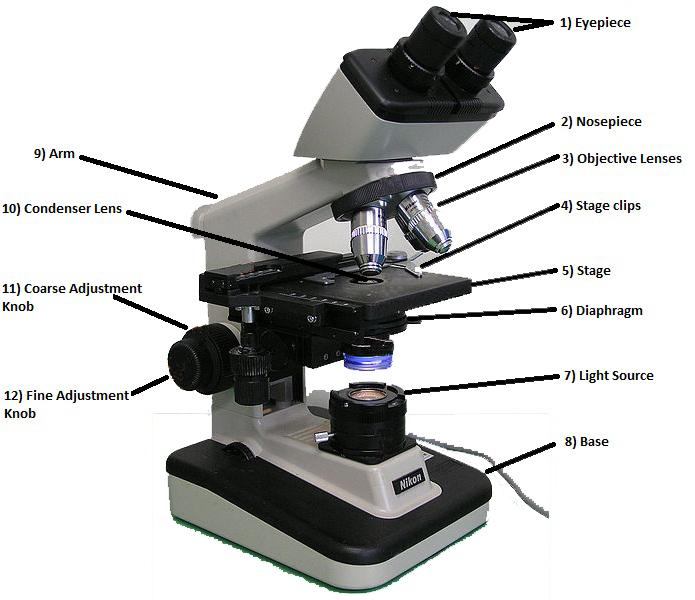 Figure 1: Different parts of a compound light microscope.
Figure 1: Different parts of a compound light microscope.
- Eyepiece: It contains the ocular lens, which provides a magnification power of 10x to 15x, usually. This is where you look through.
- Nosepiece: It holds the objective lenses and can be rotated easily to change magnification.
- Objective lenses: Usually, there are three or four objective lenses on a microscope, consisting of 4x, 10x, 40x, and 100x magnification powers. In order to obtain the total magnification of an image, you need to multiply the eyepiece lens power by the objective lens power. So, if you couple a 10x eyepiece lens with a 40x objective lens, the total magnification is 10 x 40 = 400 times.
- Stage clips: These hold the slide in place.
- Stage: It is a flat platform that supports the slide being analyzed.
- Diaphragm: It controls the intensity and size of the cone light projected on the specimen. As a rule of thumb, the more transparent the specimen, the less is the light required.
- Light source: It projects light upwards through the diaphragm, slide, and lenses.
- Base: It supports the microscope.
- Condenser lens: It helps to focus the light onto the sample analyzed. They are particularly helpful when coupled with the highest objective lens.
- Arm: It supports the microscope when carried.
- Coarse adjustment knob: When the knob is turned, the stage moves up or down, in order to coarse adjust the focus.
- Fine adjustment knob: The knob is turned to fine adjust the focus.
3. Principles of Microscopy
Understanding these concepts will help you use the microscope effectively and interpret what you see.
- Magnification: The ability to make an object appear larger.
- Total Magnification: Calculated by multiplying the magnification of the ocular lens (usually 10x) by the magnification of the objective lens being used.
- Example: Ocular (10x) × Low Power Objective (4x) = 40x Total Magnification.
- Total Magnification: Calculated by multiplying the magnification of the ocular lens (usually 10x) by the magnification of the objective lens being used.
- Resolution (Resolving Power): The ability to distinguish between two closely spaced objects as separate entities. Higher resolution means you can see finer details.
- Contrast: The difference in light intensity between the image and the adjacent background. Stains are often used to increase contrast in biological specimens.
- Field of View (FOV): The circular area visible when you look through the microscope. As magnification increases, the field of view decreases.
- Depth of Field: The thickness of the specimen that is in sharp focus at any given time. As magnification increases, the depth of field decreases. This means you’ll need to use the fine focus knob more frequently at higher magnifications to see different layers of the specimen.
4. Microscope Handling and Care
Microscopes are expensive, precision instruments. Always handle them with care.
- Carrying: Always use two hands – one hand on the arm and the other supporting the base. Carry it upright and close to your body.
- Placement: Place the microscope gently on the lab bench, away from the edge. Ensure the electrical cord is not dangling where someone could trip over it.
- Cleaning:
- Use only lens paper to clean the lenses (ocular, objectives, condenser). Never use paper towels, Kimwipes, or your shirt, as these can scratch the delicate lens coatings.
- If a lens is particularly dirty, you may need to use a special lens cleaning solution, as directed by your instructor.
- Oil Immersion Lens (100x):
- This lens requires a special type of oil (immersion oil) to be placed between the lens and the coverslip.
- Only use oil with the 100x objective lens. Never get oil on the other objective lenses (4x, 10x, 40x).
- Clean the oil from the 100x objective and the slide thoroughly with lens paper after use.
- Storage:
- Before storing, always remove the slide from the stage.
- Rotate the revolving nosepiece to the lowest power objective (usually 4x) and ensure it clicks into place.
- Lower the stage to its lowest position using the coarse adjustment knob.
- Loosely wrap the power cord around the base or designated cord holder.
- Cover the microscope with its dust cover.
5. Using the Microscope: A Step-by-Step Guide
Watch this video for a general overview of how to use a compound light microscope:
Follow these steps carefully when you use the microscope in the lab:
-
Preparation:
- Plug in the microscope and turn on the illuminator.
- Ensure the lowest power objective (e.g., 4x, often called the scanning lens) is clicked into position.
- Lower the stage completely using the coarse adjustment knob.
- Open the iris diaphragm fully or to a large aperture to start. You can adjust it later.
-
Placing the Slide:
- Place your prepared slide on the stage.
- Secure the slide with the stage clips. The coverslip should be facing up.
- Use the mechanical stage controls to move the slide so that the specimen is centered over the opening in the stage (where the light comes through).
-
Initial Focusing (Low Power):
- Looking from the side (not through the eyepiece), use the coarse adjustment knob to raise the stage as high as it will go without touching the objective lens OR until the objective lens is close to the slide.
- Now, look through the eyepiece(s). Slowly lower the stage using the coarse adjustment knob until the specimen comes into view. It might be blurry at first.
- Once you see an image, use the fine adjustment knob to bring the specimen into sharp focus.
-
Adjusting Illumination:
- Adjust the iris diaphragm to control the amount of light. Closing it slightly can increase contrast and depth of field, but too little light will make the image dim. Find the optimal balance.
- The condenser (if adjustable on your microscope) focuses the light onto the specimen. It’s usually best positioned close to the stage.
-
Increasing Magnification:
- Once the specimen is centered and in sharp focus under low power, you can switch to a higher power objective (e.g., 10x or 40x).
- Most lab microscopes are parfocal, meaning that if the specimen is in focus with one objective, it will be nearly in focus when you switch to another objective.
- Rotate the revolving nosepiece to the next higher power objective until it clicks into place.
- The image should still be visible and nearly in focus. Use only the fine adjustment knob to sharpen the focus. Never use the coarse adjustment knob with high power objectives (40x or 100x), as this can crash the lens into the slide, damaging both.
- You may need to readjust the iris diaphragm for optimal lighting at higher magnifications (usually, more light is needed).
- Re-center your specimen if needed, as your field of view is now smaller.
-
Using the Oil Immersion Lens (100x Objective):
- Only proceed if instructed and your specimen requires it.
- Focus the specimen sharply under the 40x objective and center the part you wish to view.
- Rotate the nosepiece halfway between the 40x and 100x objectives.
- Place a small drop of immersion oil directly onto the coverslip over the area you are viewing.
- Carefully rotate the 100x objective lens into the oil. The lens should make contact with the oil.
- Look through the eyepieces and use only the fine adjustment knob to focus.
- Adjust the iris diaphragm for optimal light (often fully open).
- After use: Immediately clean the oil from the 100x objective lens using lens paper. Also, clean the slide.
6. Preparing a Wet Mount Slide
A wet mount is a common way to prepare a slide of a liquid sample or a specimen suspended in liquid.
Watch this video to see how a wet mount is prepared:
Materials:
- Clean microscope slide
- Clean coverslip
- Dropper or pipette
- Specimen (liquid culture, pond water, or small piece of tissue/cells in a drop of water/stain)
- Water or stain (e.g., methylene blue)
Steps:
- Clean Slide: Start with a clean, grease-free glass slide.
- Add Specimen:
- If your specimen is liquid (e.g., pond water), place one drop in the center of the slide.
- If your specimen is solid (e.g., cheek cells, thin plant tissue), place it in the center of the slide and add a small drop of water or stain on top of it.
- Add Coverslip:
- Hold the coverslip by its edges at a 45-degree angle to the slide, with one edge touching the side of the liquid drop.
- Slowly lower the coverslip onto the drop. This technique helps to prevent air bubbles from being trapped under the coverslip.
- If you do get large air bubbles, you may need to prepare the slide again. Small air bubbles are common and can usually be ignored (they look like perfectly round circles with dark, thick edges).
- Observe: Your wet mount is now ready for observation under the microscope.
- Cleaning Up: After observation, dispose of the wet mount materials as instructed by your TA (coverslips are often disposable; slides may be washed and reused).
Staining
Sometimes, biological specimens are nearly transparent and difficult to see even with a good microscope. Stains are dyes that bind to cellular structures, increasing contrast and making them more visible. Common stains include methylene blue (stains nuclei blue) or iodine (stains starch and some cellular structures brownish-yellow). You may use these in future labs.
7. Observations and Biological Drawings
- Systematic Observation: Start at low power to get an overview. Scan the slide to find areas of interest before moving to higher magnifications.
- Biological Drawings: When you draw what you see, aim for accuracy and clarity.
- Use a sharp pencil.
- Draw large enough to show details.
- Label all relevant structures clearly using straight lines that do not cross.
- Include the specimen name, total magnification, and any stains used.
- Sometimes a scale bar is required.
8. Troubleshooting Common Issues
- Cannot see an image:
- Is the light on?
- Is the objective lens clicked into position?
- Is the diaphragm open enough?
- Is the slide centered over the light source?
- Did you start focusing with the lowest power objective?
- Is the slide upside down? (Coverslip should be up).
- Image is blurry/cannot focus:
- Are the lenses clean? (Use lens paper).
- Are you using the fine focus knob for high power?
- Is the coverslip smudged?
- If using oil immersion, is there enough oil, and is it the correct objective?
- Dark image:
- Open the diaphragm.
- Ensure the objective is fully clicked into place.
- Check the light source intensity.
- Dust or debris visible:
- Clean the eyepiece lens (it will rotate if you rotate the eyepiece).
- Clean the objective lens.
- Clean the slide/coverslip.
- Clean the condenser lens.
9. Pre-Lab Quiz
Test your understanding before you come to the lab!
This pre-lab reading should provide a solid foundation for your microscopy lab. Remember to ask your TA if you have any questions during the lab session. Happy viewing!
- Resources
- API
- Sponsorships
- Open Source
- Company
- xOperon.com
- Our team
- Careers
- 2025 xOperon.com
- Privacy Policy
- Terms of Use
- Report Issues
